Let’s review our remdesivir background for its selection as the standard COVID-19 treatment for hospitalized patients:
WATCH: Dr. Bryan Ardis – Hospital Protocols Are Killing COVID-19 Patients By Prescribing Remdesivir
Remdesivir Background
To understand the severity of this scandal, let’s briefly review some background of how Remdesivir came into use for COVID-19.
Trending: Mike Lindell: “It’s Over” — Tells Steve Bannon He Finally Has All The Evidence He’s Been Waiting On!
Remdesivir is a nucleotide analogue prodrug originally developed for the treatment of Ebola virus.
A New England Journal of Medicine study claimed that a single United States COVID-19 patient showed improvement after taking Remdesivir.
Coincidentally, the Wuhan Institute of Virology sought a patent for the use of Remdesivir.
But at the height of COVID-19, the NIH picked Remdesivir as the gold standard treatment for COVID-19.
Anthony Fraudci cited the drug’s effectiveness against Ebola as the reasoning for its use against this novel coronavirus.
Fraudci used this New England Journal of Medicine study to back his claims.
A closer look at this study below:
MORTALITY
On August 9, 2019, when 681 patients had been enrolled, the data and safety monitoring board conducted an interim analysis on data from 499 patients and, on the basis of two observations, recommended terminating random assignment to ZMapp and remdesivir.
Remdesivir was pulled from the study due to 53.1% of recipients dying from the drug.
Who supported that study?
The NIH & NIAID.
Another New England Journal of Medicine study Fraudci used to push Remdesivir as a COVID-19 treatment analyzed 53 patients from the United States, Canada, Europe, and Japan.
This is what the study found:
Seven of the 53 patients (13%) died after the completion of remdesivir treatment, including 6 of 34 patients (18%) who were receiving invasive ventilation and 1 of 19 (5%) who were receiving noninvasive oxygen support (see the Supplementary Appendix for case narratives). The median interval between remdesivir initiation and death was 15 days (interquartile range, 9 to 17).
cont.
A total of 32 patients (60%) reported adverse events during follow-up (Table 2). The most common adverse events were increased hepatic enzymes, diarrhea, rash, renal impairment, and hypotension. In general, adverse events were more common in patients receiving invasive ventilation. A total of 12 patients (23%) had serious adverse events. The most common serious adverse events — multiple-organ-dysfunction syndrome, septic shock, acute kidney injury, and hypotension — were reported in patients who were receiving invasive ventilation at baseline.
Despite these alarming studies, Remdesivir was pushed on the general public as the standard hospital protocol for COVID-19 patients.
Cheap, off-label drugs like Hydroxychloroquine (HCQ) and Ivermectin were targeted in a vicious smear campaign.
The patents for HCQ and Ivermectin expired decades ago, meaning they don’t line the pockets of pharmaceutical companies and hospital executives.
The toxicity and catastrophic kidney damage caused by Remdesivir provided the ultimate setup to blame deaths caused by the drug on COVID-19.
And the federal health agencies made a fortune buying the stock of Remdesivir, an experimental drug at the time.
The Blaze highlighted how Remdesivir is the greatest scandal of the “pandemic:”
Researchers from the Yale School of Medicine posted a preprint study in which they discovered a mutated version of SARS-CoV-2 that appears to have redeveloped in a previously infected immunocompromised woman who was treated with remdesivir. Researchers were able to sequence the genome in a way that made it clear it was related to the remdesivir use in the patient. The patient was later cured by monoclonal antibodies. “This case illustrates the importance of monitoring for remdesivir resistance and the potential benefit of combinatorial therapies in immunocompromised patients with SARS-CoV-2 infection,” the study’s authors wrote.
Obviously, this mutation appears to be a rare find, but why would we run the risk of spending $3,000 a person on a therapeutic that doesn’t work anyway if it may create immune escape?
While there's obvious corruption surrounding the use of remdesivir, the criminal activity goes much deeper than initially thought.
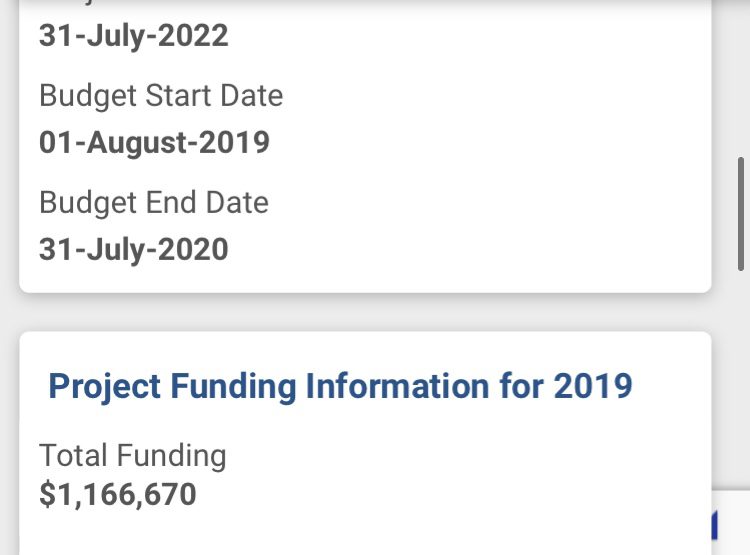
The NIH and NIAID awarded Ralph Baric of UNC Chapel Hill $1,166,670 to "accelerate the preclinical development of GS-5734" (remdesivir) for the treatment of MERS-CoV and related emerging CoV.
In partnership with Gilead Sciences, the project sought to develop approved antiviral therapies for human CoV infection.
This would "address an immediate unmet medical need and could counter future pandemic episodes."
When were the funds awarded to Baric from the NIAID?
July 15, 2019, which is several months before the first reported case of SARS-CoV-2.
That means the treatment came before the illness.
https://twitter.com/cmrqq/status/1486106813774938118
https://twitter.com/cmrqq/status/1497368254830026761
Here are screenshots from the NIH page:
As you'll notice here, the funding for Baric to develop remdesivir goes back to 2017.
The NIAID awarded Baric $1,455,240 in fiscal year 2017.
The Abstract Text states:
Project Summary Zoonotic viruses, like filoviruses and coronaviruses (CoV), represent a continuous and growing threat to global public health because they unpredictably emerge causing devastating outbreaks of pandemic disease. In the 21st century, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) emerged from zoonotic pools of viruses, causing severe disease in humans. MERS-CoV is endemic in camels in the Middle East with continuous new infections in humans. Although SARS-CoV is not currently a threat, several “prepandemic” SARS-like CoVs have been isolated from bats that replicate efficiently in human cells and are resistant to existing therapies. With the unpredictable overlap of human and wild animal ecologies, the potential for novel CoV emergence into humans is highly probable. Currently, there are no approved antiviral therapies for any human CoV infection. Broad-spectrum CoV therapies that control known human and zoonotic CoV infections would address an immediate unmet medical need and could counter future pandemic episodes. In partnership with Gilead Sciences, we have demonstrated that the nucleoside prodrug, GS-5734, is highly efficacious in inhibiting multiple human and zoonotic CoV in vitro and SARS-CoV in vivo. The primary goal of our program is to accelerate the preclinical development of GS-5734 and promote IND licensure for the MERS-CoV indication. To thoroughly evaluate the breadth of antiviral activity and predict efficacy against future emerging CoV, we will also assess efficacy against a panel of CoV representative of family-wide genetic diversity, including prepandemic zoonotic strains poised for emergence. Focusing on the highly pathogenic MERS-CoV, our unique partnership integrates: i) metagenomics and recombinant virus synthetic genome recovery, ii) primary human lung cell models, iii) cutting edge virology and biochemistry, iv) robust murine and primate models of human disease and v) state of the art metabolic and pharmacokinetic analysis. In Aim 1, we refine the pharmacokinetics, pharmacodynamics and breadth of GS-5734 through efficacy and metabolism studies in various primary human cells with a diverse array of human and zoonotic CoV and through the evaluation of in vivo efficacy in murine and non-human primate models of MERS- and SARS-CoV. In Aim 2, we select for resistance against SARS-CoV and MERS- CoV, and determine the effect of resistance on virus replication, fitness and susceptibility to treatment. In Aim 3, we determine if the mechanism of action of GS-5734 is a result of direct effects on viral RNA replication and/or alteration of antiviral immunity via deep sequencing and single molecule RNA fluorescence in situ hybridization of vehicle or drug treated infected cells and mice. We articulate a development strategy for broad- spectrum therapeutics that could be extended to a multitude of emerging viral pathogens threatening global public health.
Public Health Relevance Statement:
Project Narrative In partnership with Gilead Sciences, we aim to accelerate the preclinical development of GS-5734 and promote IND licensure. We define the pharmacokinetics, pharmacodynamics, resistance profile, efficacy breadth and mechanism of action of GS-5734 against MERS-CoV and related emerging CoV.
And during the whole "pandemic," remdesivir has remained the only FDA-approved antiviral treatment for COVID-19.








Join the conversation!
Please share your thoughts about this article below. We value your opinions, and would love to see you add to the discussion!