Remember sitting in your car, sometimes for hours, to get tested for COVID?
Remember jamming that long q-tip up your nose to the point where it tickled your brain and brought tears to your eyes?
Remember doing all of that over and over again?
Well, I didn’t submit to that crap, but I know millions of Americans did…for over two years and counting!
Guess what?
It might have all been a sham.
At the very least, it now looks as though the test was woefully inadequate and may likely have led to thousands or millions of false positives.
Why do I say that?
Well, I don’t….the CDC just did!
Yes folks, the CDC has now discontinued the PCR test because it can’t differentiate between COVID and the flu.
Take a look:
On Dec 31, 2021, the CDC will withdraw the use of the PCR test for covid testing. They’ve finally admitted the test doesn’t differentiate between the flu & the Corona! Hmm that’s why the flu has been eradicated for the last 2 yrs? Sorry CDC we were aware & so were you! Liars!
— Domenica D'Elia (@domenicadelia22) December 29, 2021
CDC withdraw use apparently *not* down to fact that it CANNOT distinguish between flu & C19, but as there's no longer much call for the tests – Mmm but US topped best part of half a mill + tests in one day?
MMS are inventing a handy explanation it seems … Open your eyes FGS!!? pic.twitter.com/vB0lw6yoDd
— Zor ✨ (@zorrozom) December 30, 2021
This comes directly from the CDC’s own website:
After December 31, 2021, CDC will withdraw the request to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel, the assay first introduced in February 2020 for detection of SARS-CoV-2 only. CDC is providing this advance notice for clinical laboratories to have adequate time to select and implement one of the many FDA-authorized alternatives.
The CDC website also confirms the reason for the change:
In preparation for this change, CDC recommends clinical laboratories and testing sites that have been using the CDC 2019-nCoV RT-PCR assay select and begin their transition to another FDA-authorized COVID-19 test. CDC encourages laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses. Such assays can facilitate continued testing for both influenza and SARS-CoV-2 and can save both time and resources as we head into influenza season. Laboratories and testing sites should validate and verify their selected assay within their facility before beginning clinical testing.
In other words, the PCR couldn’t do that!
Folks, how many false positives did we have over the past 2+ years?
Was this “pandemic” all based on a normal seasonal cold and flu season?
Did we destroy the economy and cripple the supply chain over….the flu?
It’s beginning to look like it.
Oh, and just in case they change that website, let’s screenshot it for journalistic purposes, shall we?
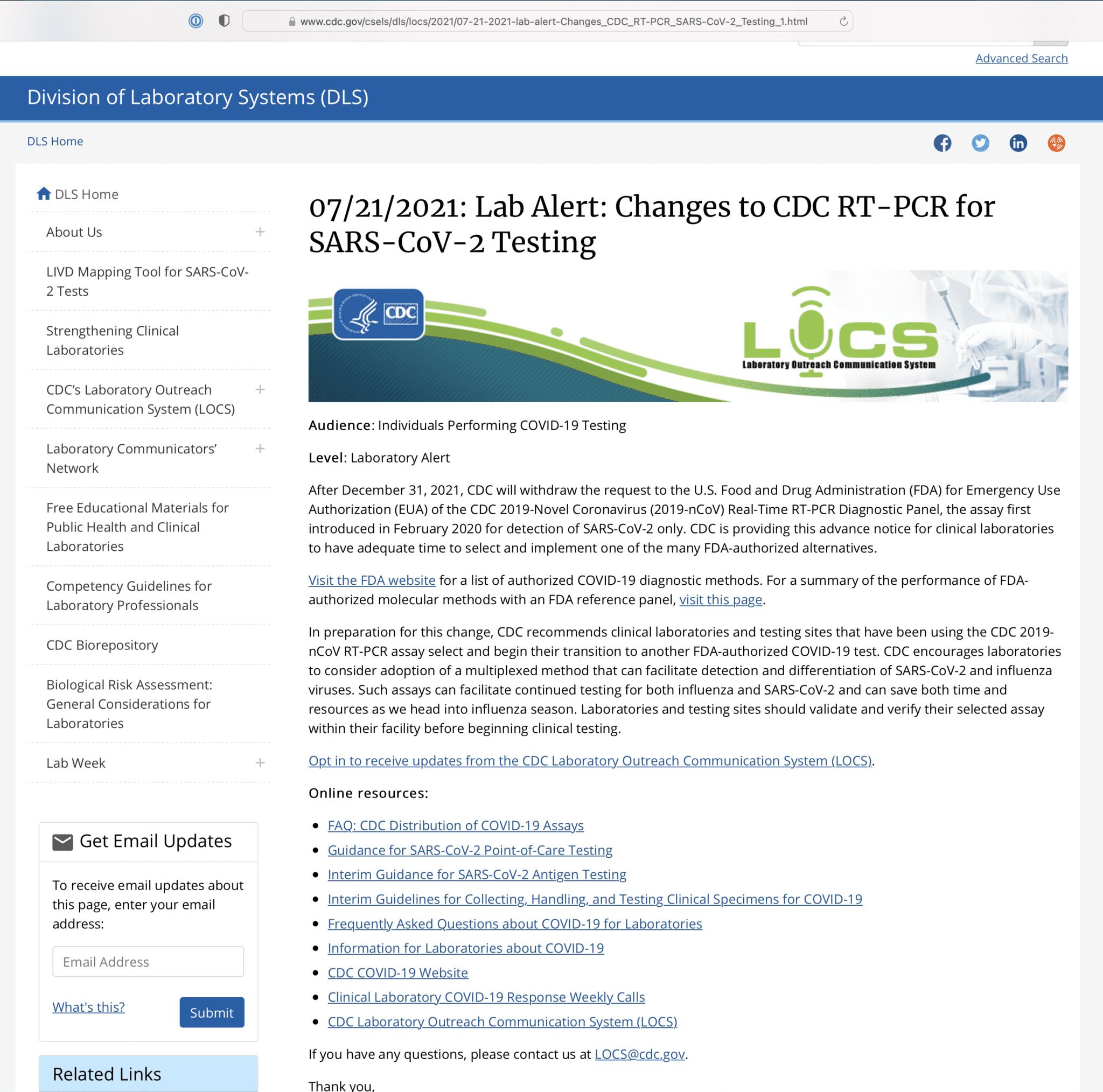
What else do you notice in that screenshot?
Do you notice the date?
Published 7/21/2021.
And when are they pulling the PCR test?
Not until 12/31/21.
I guess they needed higher numbers these last 6 months to keep scaring people into getting the jab?
But that would be….conspiracy theory!
Many online are now saying it flat out: was this whole thing a total fraud?
December 31, 2021 – CDC to WITHDRAW Emergency Use Authorization for PCR Tests Used to Detect – Epidemic was Total FRAUD https://t.co/UJJoXj5Rb1
— Lauretta (@Tanfox13) January 1, 2022
The American Association for Clinical Chemistry (AACC) has confirmed the report as well:
After December 31, 2021, the Centers for Disease Control and Prevention (CDC) will withdraw its request to the Food and Drug Administration (FDA) for emergency use authorization (EUA) of the CDC 2019-Novel Coronavirus Real-Time RT-PCR diagnostic panel. CDC is announcing this in advance so that any clinical laboratories that are using this test have adequate time to select and implement one of the many FDA-authorized alternatives.
CDC first introduced this assay for detection of SARS-CoV-2 in February 2020. Now, however, the agency has decided to voluntarily withdraw its request for EUA primarily because “hundreds of SARS-CoV-2 [polymerase chain reaction (PCR)] tests have received EUAs and are widely available,” said AACC President Stephen R. Master, MD, PhD, FAACC. This means that “CDC’s test is no longer needed to fill an unmet testing need.”
As laboratories look for an alternative to CDC’s test, the agency encourages labs to consider adopting a multiplexed method that detects and differentiates between SARS-CoV-2 and influenza viruses. This will facilitate a more cost-efficient approach to testing once flu season arrives, according to Master. CDC actually has a separate EUA for such a test—one that detects and distinguishes between SARS-CoV-2, influenza A, and influenza B—that it will be maintaining. Moving forward, CDC and other public health laboratories plan to use this multiplex test so they can simultaneously monitor flu activity in addition to SARS-CoV-2.
So, if you lost your job, or jammed dozens of nasal swabs into your brain, or if you got jabbed under coercion or threat, my question is this: Was it all based on phony data?
Was it all a fraud?
Is the curtain finally being pulled back on what can only be described as an attack on the American people?
We’ll continue to cover this developing story…



Join the conversation!
Please share your thoughts about this article below. We value your opinions, and would love to see you add to the discussion!