While mainstream media pushes for war with Russia, the explosive Pfizer document release remains unnoticed by the masses.
WeLoveTrump previously reported the 9 pages of side effects listed in one of the documents.
After digging deeper into the files, the levels of corruption amongst Pfizer, BioNTech, and the FDA have rose to the surface.
One of the most incriminating discoveries thus far is a document titled "Prescription Drug User Fee Payment."
According to the file, BioNTech paid the FDA $2,875,842.00 on 4/20/2021 for the “COMIRNATY COVID-19 mRNA Vaccine."
The FDA subsequently approved the experimental injection in August of 2021.
Another intriguing find was a document called "EXTERNAL DATA MONITORING COMMITTEE."
Health Impact News explained the stated purpose of the document:
This External Data Monitoring Committee (E-DMC) (hereafter referred to as “the committee”) is a single, external, independent, expert advisory group established to oversee safety and efficacy data from the BNT162 Vaccine Program. The primary rationale for establishing the committee is to make certain that appropriate external safeguards are in place to help ensure the safety of subjects and to maintain scientific rigor and study integrity while the trial is on-going.
The committee will review accumulating safety data across all studies, as well as efficacy data in the Phase 2/3 portion of the C4591001 study. The committee will advise Pfizer regarding the safety of current participants and those yet to be recruited, as well as the continuing scientific validity of the trial. In addition to safety review by the committee, qualified Pfizer personnel will review safety data as specified in the safety surveillance review plan and will inform the committee of significant findings. Efficacy data from the C4591001 study will be available to the committee when there is a planned interim analysis of efficacy or if this is considered necessary to conduct a risk-benefit assessment.
The committee is tasked with properly ensuring "the safety of subjects and to maintain scientific rigor."
While you'd think the FDA would have this task, that's an incorrect assumption.
Pfizer was responsible for conducting the study and BioNTech was the regulatory sponsor of the study.
As stated in Section 1. Introduction:
Pfizer is responsible for conducting this study. BioNTech is the regulatory sponsor of this study.
As stated above, the committee is supposed to consist of external, independent members to oversee safety and efficacy data.
You'd think the FDA would have the responsibility of ensuring no "conflicts of interest."
But again, you'd be wrong with that assumption.
Section 2.1. Conflicts of Interest reads:
The committee members will complete a CT22-GSOP-RF01 Independent Oversight Committee Member Conflict of Interest Form. Committee members should be free of apparent significant conflicts of interest. Any potential conflict of interest that develops during a member’s tenure on the committee must be disclosed by the committee member. Pfizer will determine if any potential conflict requires termination of committee membership.
Each time the committee meets, the study team will ask the committee members to consider whether or not any changes in their conflict of interest status have emerged. Status must be recorded in the committee open meeting minutes and any potential conflicts must be reported using CT22-GSOP-RF01.
Did the FDA play any role in monitoring the data of the COVID-19 injection clinical trials?
It appears the "regulatory agency" signed off on a process conducted by Pfizer and funded by BioNTech.
Here are the members of the “External Data Monitoring Committee” that were, according to this document, chosen, monitored, and investigated by Pfizer to conclude there were no "conflicts of interest."
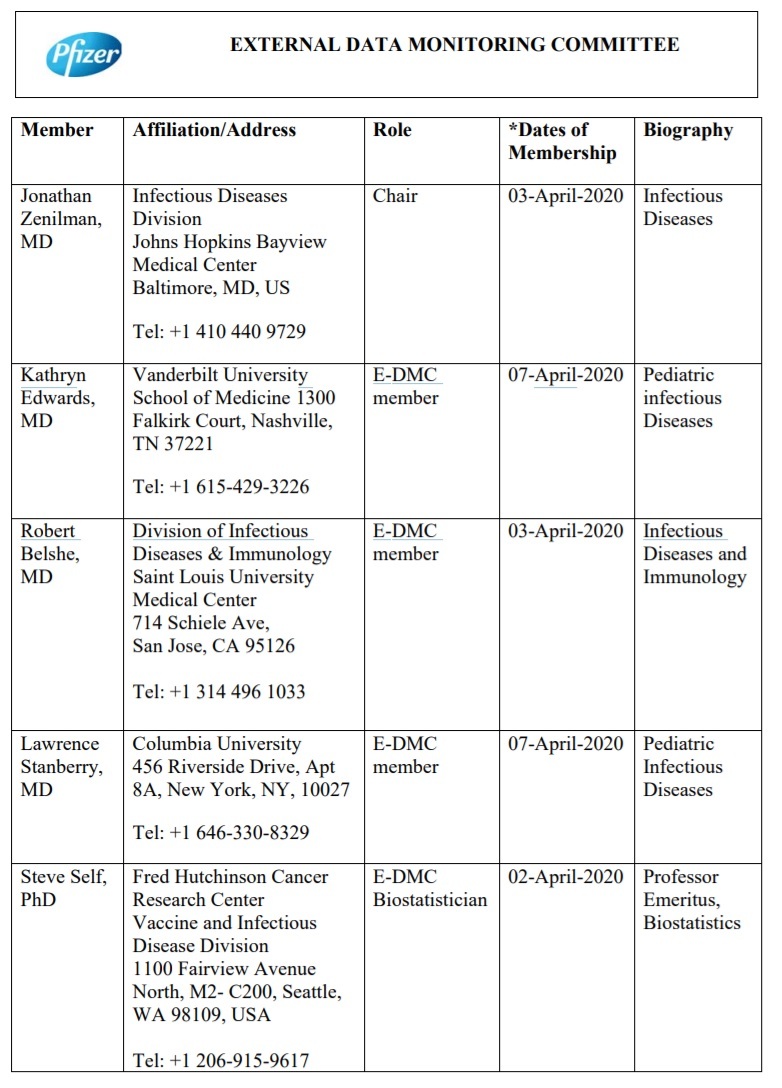
Health Impact News noted that several individuals compiling the data for this committee to review were located in China:
Rong Zhang: Senior Statistical Programming Lead
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Rong.Zhang@pfizer.comChen Xu*: Senior Statistical Programmer
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Chen.Xu4@pfizer.comHuan Liu* Senior Statistical Programmer
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Huan.Liu@pfizer.comJiyang Chen*: Senior Statistical Programmer
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Jiyang.Chen@pfizer.comBochen Zhu*: Senior Statistical Programmer
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Bochen.Zhu@pfizer.comRan Xiong*: Senior Statistical Programmer
4/F, Building 3, Lotus Business Park, Lane
60, Naxian Road,
Pudong ZhangJiang Hi-tech. Park, Shanghai,
China, 201203
Ran.Xiong@pfizer.comI wonder if the raw data is also located in China?
You can review all the Pfizer documents HERE.




Join the conversation!
Please share your thoughts about this article below. We value your opinions, and would love to see you add to the discussion!