VAERS (Vaccine Adverse Event Reporting System) is our complex, inefficient, and underreported system used to track potential side effects of vaccines.
Although the reporting system is by no means perfect, it’s currently our only method to detect vaccine-related injuries.
The dramatic rise in VAERS reports during 2021 should present a major red flag for the experimental COVID-19 injections.
Deaths in Vaccine Adverse Event Reporting System (VAERS), since its start in 1990.
2021 is off the chartshttps://t.co/CNiza9ePMl pic.twitter.com/1SSETps4lg
— RarefiedMD (@DenseMD) April 1, 2021
Trending: Yale Students Say Anonymous Reporting System Has Turned School Into Orwellian ‘Surveillance State’
OpenVAERS is a private entity that takes the publicly available data and makes it more user-friendly.
The latest VAERS data through 11/5/2021 showed these reported figures:
New #VAERS data for the week now available on #OpenVAERS through Nov. 5, 2021. https://t.co/OVL3IZu4t5@stkirsch identifies the URF (underreporting factor) as 41. pic.twitter.com/GJeLHmJ30w
— OpenVAERS (@OpenVAERS) November 12, 2021
While OpenVAERS simplifies reading the data, I’ll show you how to navigate the VAERS data on the CDC website.
It’s called CDC Wonder, and the tool helps users interpret Public Health data and information.
After using the CDC’s search engine, the results are more shocking than OpenVAERS indicates.
But first, it’s fair to recognize the limitations of the VAERS data.
From the CDC:
- VAERS data are derived from a passive surveillance system and represent unverified reports of health events, both minor and serious, that occur after vaccination.
- Such data are subject to limitations of under-reporting, simultaneous administration of multiple vaccine antigens (making it difficult to know to which of the vaccines, if any, the event might be attributed), reporting bias, and lack of incidence rates in unvaccinated comparison groups.
- While some events reported to VAERS are truly caused by vaccines, others may be related to an underlying disease or condition, to drugs being taken concurrently, or may occur by chance shortly after a vaccine was administered.
- VAERS occasionally receives case reports from US manufacturers that were reported to their foreign subsidiaries. Under FDA regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and unexpected (in other words, it does not appear in the product labeling), they are required to submit it to VAERS. It is important to realize that these case reports are of variable data quality and completeness, due to the many differences in country reporting practices and surveillance system quality.
- In some media reports and on some web sites on the Internet, VAERS reports are presented as verified cases of vaccine deaths and injuries. Statements such as these misrepresent the nature of the VAERS surveillance system.
The coding of VAERS reports also requires careful interpretation:
- All narrative text taken from VAERS reports are coded and entered into the VAERS database utilizing the FDA’s Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) which are key words representing the medical condition(s) described in the case report.
- Establishing causal relationships between vaccines and adverse events requires additional scientific investigation.
- The CDC and FDA take into account the complex factors mentioned above, and others, when monitoring vaccine safety and analyzing VAERS reports.
The purpose of VAERS is to detect possible signals of adverse events associated with vaccines. Additional scientific investigations are almost always required to properly validate signals from VAERS and establish a cause and effect relationship between a vaccine and an adverse event. For example, potential concerns raised by VAERS are investigated through a CDC project called the Vaccine Safety Datalink (VSD). VSD is a large-linked database and includes information on more than six million people, allowing for planned vaccine safety studies as well as timely investigations of hypotheses.
VAERS is meant to signal the public about potential adverse events with vaccines and investigate further before more damage is done.
The reporting system has indicated major warning signs for the experimental COVID-19 injections.
Yet, health officials and governments push forward to inject the global population.
CDC Wonder Breakdown:
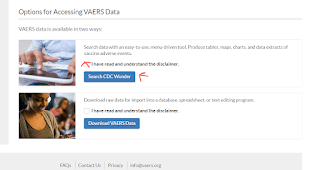
Start by going to https://vaers.hhs.gov/data.html
Scroll down here and check the disclaimer then click “Search CDC Wonder”
Scroll down here and click “I agree”
It will look as if the page refreshed but you will notice the 2 grayed out boxes can now be clicked. Click “VAERS Data Search”
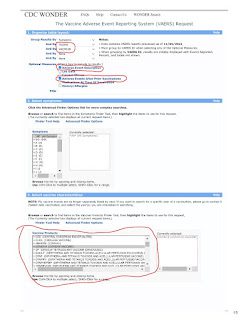
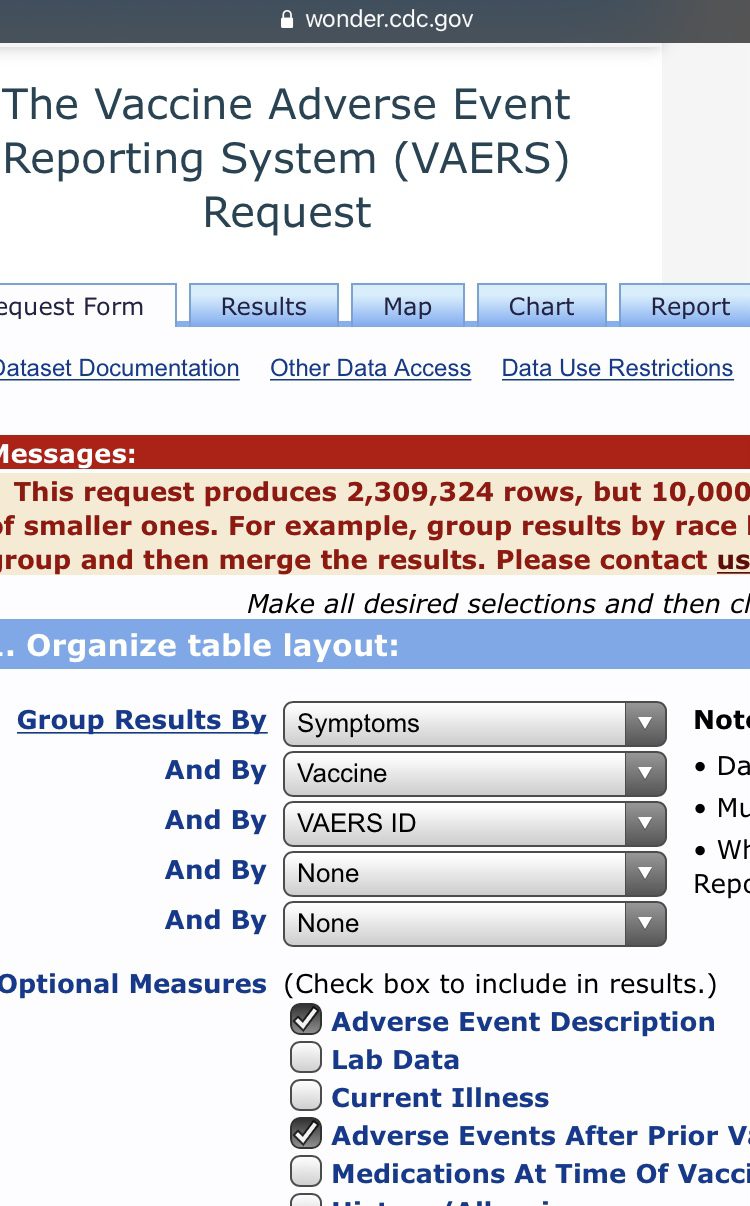
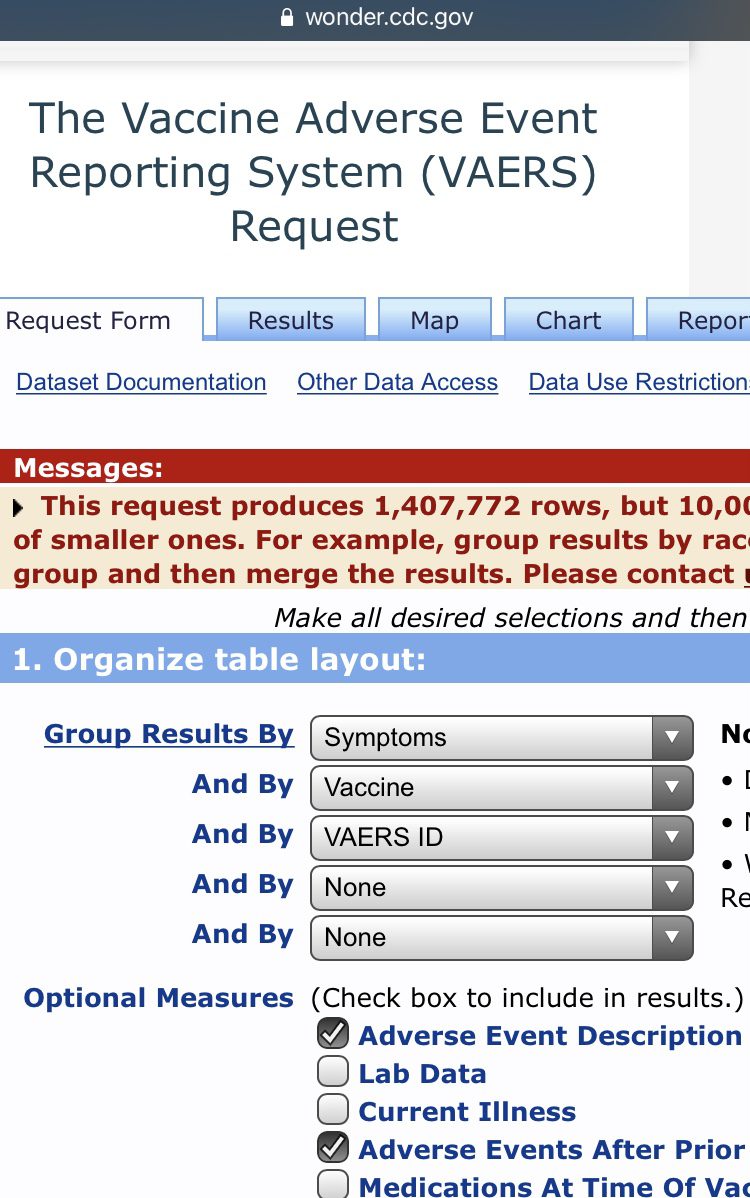
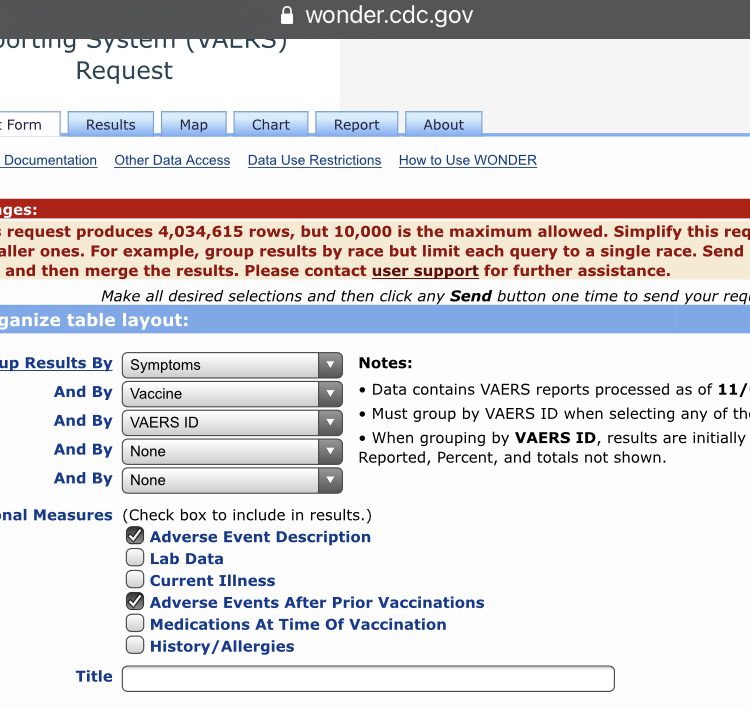
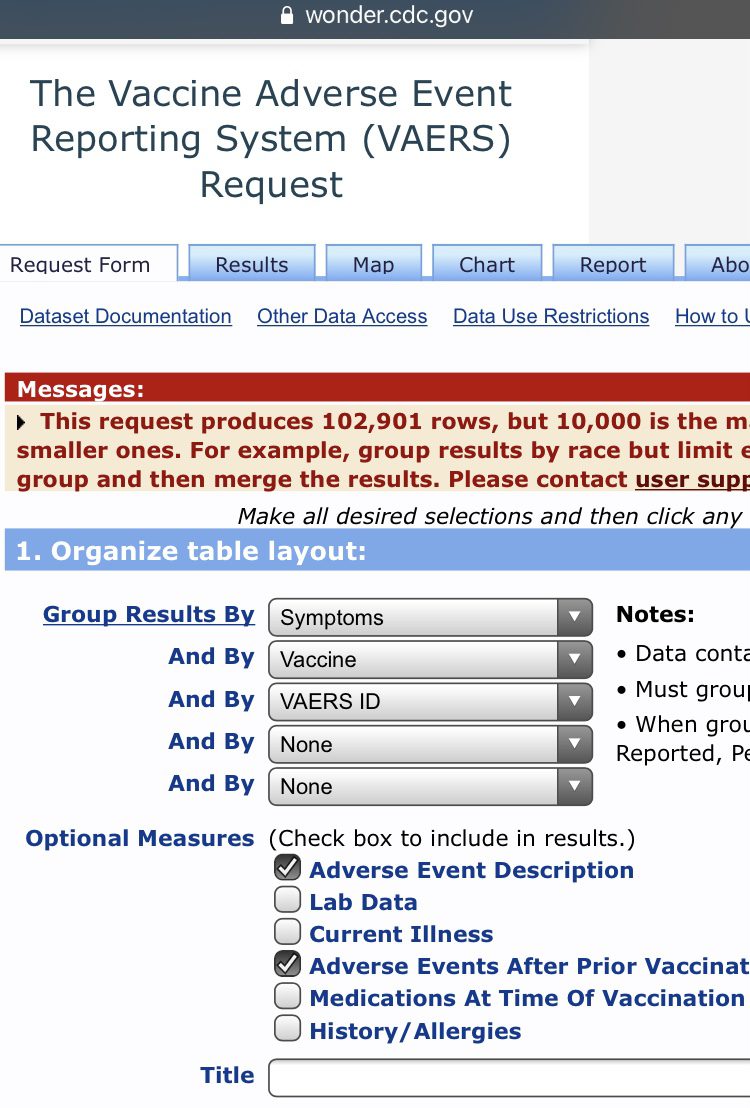
While you can make the search results more specific, organize table layout by symptoms, vaccine, and VAERS ID. In the optional measures, tick the Adverse Event Description and Adverse Events After Prior Vaccinations boxes. Now depending on your search you may get a very large number. If it exceeds 10,000 then you will get a popup in a yellow box that contains your results but will also say “but 10,000 is the maximum allowed.” This is because VAERS won’t break down over 10,000 cases to you. To access the actual cases just tighten the search, i.e. select ages, location etc.
In section 3 (Select vaccine characteristics), choose COVID-19 Vaccine:
Now, I’ll show you some results after a few searches in this VAERS database.
Like the OpenVAERS data, this also includes the “nondomestic” reports.
Below are the numbers I ran at the time of writing on 11/16/2021:
Pfizer/BioNTech – All Events
2,309,324
Pfizer/BioNTech – Deaths
69,906
Moderna – All Events
1,407,772
Moderna – Deaths
25,847
All Manufacturers – All Events
4,034,615
All Manufacturers – Deaths
102,901
And these are the reported adverse events and deaths in the VAERS system.
Prior estimates indicate around 1-10% of vaccine adverse events are reported to VAERS.
From National Vaccine Information Center:
During the meeting of the National Vaccine Advisory, CDC officials reported that about 70 percent of VAERS reports are handwritten and submitted by mail or fax, while 30 percent are online submissions.4 The system receives about 30,0005 reports annually and it is estimated that only between one to 10 percent of vaccine adverse events are reported to VAERS.6, 7, 8 9Underreporting of vaccine reactions in the U.S. is a widely acknowledged weakness of VAERS.10 It is also known that little has been done by federal health officials to increase vaccine provider reporting to VAERS since the passage of the National Childhood Vaccine Injury Act in 1986, which requires the reporting of vaccine adverse events.
Although the new reporting form proposed by the CDC adds new features while retaining many of current form’s data collection fields, NVIC opposes the CDC’s proposal to eliminate the option of submitting handwritten reports. The proposed shift to a completely “paperless” system is likely to result in even more underreporting of vaccine adverse events and penalize those who are not computer literate. These changes could also hinder the effective monitoring and detection of unusual vaccine adverse events occurring in the general population by health scientists.






Join the conversation!
Please share your thoughts about this article below. We value your opinions, and would love to see you add to the discussion!